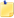Table 3: Major Plastic Resins and Their Uses
Resin CodeResin NameCommon UsesExamples of Recycled Products
Polyethylene Terephthalate (PET or PETE)
Soft drink bottles, peanut butter jars, salad dressing bottles, mouth wash jars
Liquid soap bottles, strapping, fiberfill for winter coats, surfboards, paint brushes, fuzz on tennis balls, soft drink bottles, film
High density Polyethylene (HDPE)
Milk, water, and juice containers, grocery bags, toys, liquid detergent bottles
Soft drink based cups, flower pots, drain pipes, signs, stadium seats, trash cans, re-cycling bins, traffic barrier cones, golf bag liners, toys
Polyvinyl Chloride or Vinyl (PVC-V)
Clear food packaging, shampoo bottles
Floor mats, pipes, hoses, mud flaps
Low density Polyethylene (LDPE)
Bread bags, frozen food bags, grocery bags
Garbage can liners, grocery bags, multi purpose bags
Polypropylene (PP)
Ketchup bottles, yogurt containers, margarine, tubs, medicine bottles
Manhole steps, paint buckets, videocassette storage cases, ice scrapers, fast food trays, lawn mower wheels, automobile battery parts.
Polystyrene (PS)
Video cassette cases, compact disk jackets, coffee cups, cutlery, cafeteria trays, grocery store meat trays, fast-food sandwich container
License plate holders, golf course and septic tank drainage systems, desk top accessories, hanging files, food service trays, flower pots, trash cans
 ChemPaths UW-Madison on 26 Jan 09Hi!
ChemPaths UW-Madison on 26 Jan 09Hi! vediphile on 02 Jul 22Atomic and Ionic Radii | Boron Family | P-Block Elements | IIT-JEE NEET youtube.com/watch?v=fqK9e_jqKLU
vediphile on 02 Jul 22Atomic and Ionic Radii | Boron Family | P-Block Elements | IIT-JEE NEET youtube.com/watch?v=fqK9e_jqKLU