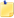The Covalent Bond - 0 views
-
The orbital is centered between the two atoms, so electron density is highest there. Again, as said above, this reduced the total energy of the system, and electrons in this orbital will thus lead to a covalent bond between the two atoms. This orbital is referred to as a bonding orbital.b)With the LUMO molecular orbital of H2 there is zero electron density between the two hydrogen atoms. There is a node in the orbital exactly the two atoms.
-
 Michael Boykin on 14 Sep 09Isn't this a contradiction? The top portion says the highest density is between the two atoms, and the bottom part says there is zero electron density between the two atoms...
Michael Boykin on 14 Sep 09Isn't this a contradiction? The top portion says the highest density is between the two atoms, and the bottom part says there is zero electron density between the two atoms... -
 ChemPaths UW-Madison on 14 Sep 09In part (b), the question asks about the LUMO - or the Lowest (Energy) Unoccupied Molecular Orbital. It is where the 3rd electron would go, if you tried to put it into this molecule. Thus what the last sentence in this solution states: "If electrons occupied this orbital, it would lead to the dissolution of the covalent bond." Does that make sense?
ChemPaths UW-Madison on 14 Sep 09In part (b), the question asks about the LUMO - or the Lowest (Energy) Unoccupied Molecular Orbital. It is where the 3rd electron would go, if you tried to put it into this molecule. Thus what the last sentence in this solution states: "If electrons occupied this orbital, it would lead to the dissolution of the covalent bond." Does that make sense?
-